Ancient Retrovirus Embedded In Human Genome Helps Fight HIV-1 Infection
Throughout our evolution, viruses have continually infected humans just as they do today. Some early viruses became integrated into our genome and are now known as human endogenous retroviruses (HERVs). Over millions of years, they became inert due to mutations or major deletions in their genetic code. Today, one of the most studied HERV families is the HERV-K family, which has been active since the evolutionary split of humans and chimpanzees with some members perhaps actively infecting humans within the past couple hundred thousand years.
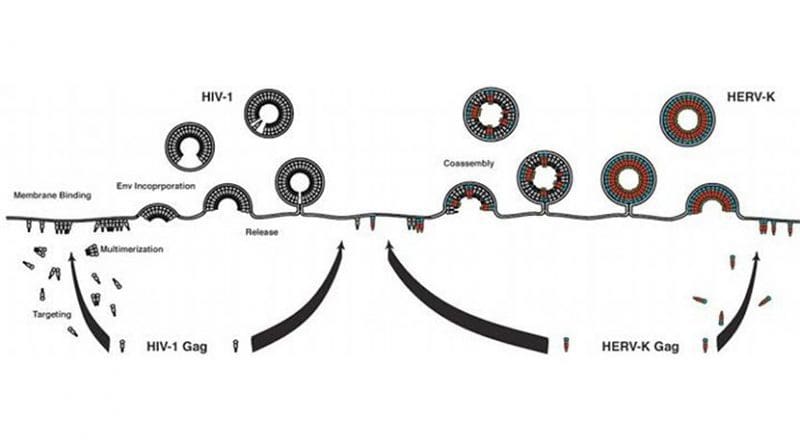
HERVs have become a target for HIV researchers because studies have shown that T-cells produce an immune response against HERVs in those infected with HIV. It is now thought that HERV expression can be caused by HIV infection and that HIV would become an easier target by aiming at the HERV antigens rather than the ever-mutating HIV antigens. Following that idea, previous research from Kumamoto University in Japan revealed an apparent correlation between the coassembly of HIV-1 group specific antigen (Gag) and HERV-K Gag, and the reduced particle proliferation and infectivity of HIV-1. In their current study, the researchers sought to clarify how HERV-K Gag affects HIV-1 in this manner.
They reported that HERV-K Gag changes the size and morphology of progeny HIV-1 particles during the early stages of coassembly. This occurs because the HERV-K Gag capsid (CA), i.e. the virus protein shell, colocalizes (overlaps) partially with HIV-1 Gag at the plasma membrane. This also results in a reduced number of mature HIV-1 particles and lower HIV-1 release and infectivity.
“While we have found that release efficiency and infectivity of HIV-1 particles are hindered by HERV-K Gag,” said project leader, Dr. Kazuaki Monde, “they appear to be products of two separate mechanisms. HIV-1 particle release from cells that also express HERV-K Gag is reduced significantly but the specifics of how infectivity is also reduced still eludes us. Certainly, more research into HERV-K CA is needed to determine how it is able to reduce both particle release and infectivity of HIV-1.”