Blood Plasma Therapy And Treatment Of COVID-19 – Analysis
Plasma is the largest part of your blood. It, makes up more than half (about 55%) of its overall content. When separated from the rest of the blood, plasma is a light yellow liquid. Plasma carries water, salts and enzymes. The main role of plasma is to take nutrients, hormones, and proteins to the parts of the body that need it. Cells also put their waste products into the plasma. The plasma then helps remove this waste from the body. Blood plasma also carries all parts of the blood through your circulatory system.
In addition plasma also contains other important compositions that include antibodies, clotting factors, and the proteins albumin and fibrinogen and when we donate blood, healthcare providers can separate these vital parts from your plasma. These parts can then be concentrated into various products. These products are then used as treatments that can help save the lives of people. It plays a vital role in an intravascular osmotic effect that keeps electrolyte concentration balanced and protects the body from infection and other blood disorders.
Fresh frozen plasma is on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system. It is of critical importance in the treatment of many types of trauma which result in blood loss, and is therefore kept stocked universally in all medical facilities capable of treating trauma or that pose a risk of patient blood loss.
Therefore, given the public health emergency that the COVID-19 pandemic presents, while clinical trials are being conducted and an expanded access protocol is available. At a small scale doctors are facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections through the process of the patient’s physician requesting a single patient emergency.
Facts about blood plasma
The proteins and antibodies in plasma are also used in therapies for rare chronic conditions. These include autoimmune disorders and hemophilia. People with these conditions can live long and productive lives because of the treatments. In fact, some health organizations call plasma “the gift of life.”
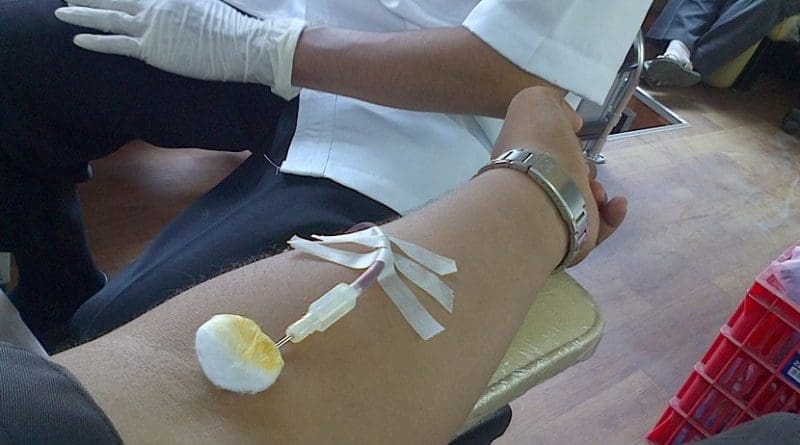
If we want to donate plasma to help others in need, we will go through a screening process. This is to make sure that our blood is healthy and safe and if we qualify as a plasma donor, we will spend about an hour and a half at a clinic on every follow-up visit.
During the actual blood donation process, your blood is drawn through a needle placed in a vein in one arm. A special machine separates the plasma and often the platelets from your blood sample. This process is called plasmapheresis. The remaining red blood cells and other blood components are then returned to your body, along with a little saline (salt) solution.
People with the blood type AB are in the greatest demand for plasma donation. They make up just 2 in 50 people, their plasma is universal. This means their plasma can be used by anyone. At noncommercial donation sites, people can donate plasma every 28 days, up to 13 times a year.
As said above, plasma is the largest part of our blood. It, makes up more than half (about 55%) of its overall content. When separated from the rest of the blood, plasma is a light yellow liquid. Plasma carries water, salts and enzymes. The main role of plasma is to take nutrients, hormones, and proteins to the parts of the body that need it. Cells also put their waste products into the plasma. The plasma then helps remove this waste from the body. Blood plasma also carries all parts of the blood through your circulatory system.
Role and investigation of blood plasma
Plasma is a critical part of the treatment for many serious health problems. This is why there are blood drives asking people to donate blood plasma. One investigational treatment being explored for COVID-19 is the use of convalescent plasma collected from individuals who have recovered from COVID-19. Convalescent plasma that contains antibodies to severe acute respiratory syndrome coronavirus 2 or SARS-CoV-2 (the virus that causes COVID-19) is being studied for administration to patients with COVID-19.
The use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2003 SARS-CoV-1 epidemic, the 2009-2010 H1N1 influenza virus pandemic, and the 2012 MERS-CoV epidemic.
Although promising, convalescent plasma has not yet been shown to be safe and effective as a treatment for COVID-19. Investigators wishing to study the use of convalescent plasma in a clinical trial should submit requests to FDA for investigational use under the traditional IND regulatory pathway (21 CFR Part 312). CBER’s Office of Blood Research and Review is committed to engaging with sponsors and reviewing such requests expeditiously.
Under the process application for expanded access is an alternative for use of COVID-19 convalescent plasma for patients with serious or immediately life-threatening COVID-19 disease who are not eligible or who are unable to participate in randomized clinical trials.
The FDA has worked with multiple federal partners and academia to open an expanded access protocol to facilitate access to COVID-19 convalescent plasma across the nation. Then only related comfort is made available through participation of acute care facilities in an investigational expanded access protocol.
At a small scale doctors are facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections through the process of the patient’s physician requesting a single patient emergency.
As a further step health care providers or acute care facilities seeking to use COVID-19 convalescent plasma should include information in the IND submission that the COVID-19 convalescent plasma will be obtained from an FDA-registered blood establishment that follows the donor eligibility criteria and donor qualifications described in collecting plasma from donors.
Other precautions in blood plasma therapy
Complete resolution of symptoms at least 14 days prior to donation, AND Negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. Male donors, or female donors who have not been pregnant, or female donors who have been tested since their most recent pregnancy and results interpreted as negative for HLA antibodies.
When measurement of neutralizing antibody titers is available, it is recommended to neutralise antibody titers of at least 1:160. When measurement of neutralizing antibody titers is not available, consider storing a retention sample from the convalescent plasma donation for determining antibody titers at a later date.
Registered and licensed blood establishments that collect plasma intended for transfusion do not need to request a supplement to their license or obtain their own IND to collect and manufacture COVID-19 convalescent plasma for investigational use provided they 1) follow their standard operating procedures for plasma collection and all applicable regulations, and 2) collect plasma from individuals that meet the donor qualifications specified above, which would be included in the applicable IND(s) held by a health care provider or other sponsor.
Once manufactured, the COVID-19 convalescent plasma may be distributed for investigational use. FDA recognises that the current circular of information does not contain specific information about COVID-19 convalescent plasma regarding indications for use, dosage information, contraindications or cautions, but it provides information on the use of plasma.